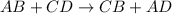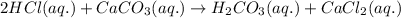Chemistry, 22.11.2019 04:31 morgannwaldrupp
To measure the amount of calcium carbonate (caco) in a seashell, an analytical chemist crushes a 2.100 g sample of the shell to a fine powder and titrates it to the endpoint with 119. ml of 0.300 m hydrogen chloride (hci) solution. the balanced chemical equation for the reaction is 2hci(aq) + co, (aq) ? h2co3(aq) + 2a (aq). what kind of reaction is this?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1


Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 22.06.2019 22:30
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1
You know the right answer?
To measure the amount of calcium carbonate (caco) in a seashell, an analytical chemist crushes a 2.1...
Questions


History, 17.01.2020 09:31




Biology, 17.01.2020 09:31

Biology, 17.01.2020 09:31

Mathematics, 17.01.2020 09:31


History, 17.01.2020 09:31

Computers and Technology, 17.01.2020 09:31

Mathematics, 17.01.2020 09:31



Mathematics, 17.01.2020 09:31

Mathematics, 17.01.2020 09:31

Mathematics, 17.01.2020 09:31



Computers and Technology, 17.01.2020 09:31