1. calculate the actual yield of magnesium oxide for each trial.
trial 1:
trial...

Chemistry, 22.11.2019 04:31 cloeybrown
1. calculate the actual yield of magnesium oxide for each trial.
trial 1:
trial 2:
2. magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial.
trial 1:
trial 2:
3. determine the percent yield of mgo for your experiment for each trial.
trial 1:
trial 2:
4. determine the average percent yield of mgo for the two trials.
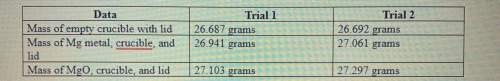

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 19:40
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests.which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3

Chemistry, 23.06.2019 05:50
Which of the following isotopes has the same number of neutrons as phosphorus-31?
Answers: 1
You know the right answer?
Questions

Health, 20.04.2021 18:10

Mathematics, 20.04.2021 18:10

Mathematics, 20.04.2021 18:10

Mathematics, 20.04.2021 18:10

English, 20.04.2021 18:10

Mathematics, 20.04.2021 18:10

Physics, 20.04.2021 18:10

Mathematics, 20.04.2021 18:10

English, 20.04.2021 18:10

Mathematics, 20.04.2021 18:10

Mathematics, 20.04.2021 18:10

Mathematics, 20.04.2021 18:10




Mathematics, 20.04.2021 18:10




Social Studies, 20.04.2021 18:10



