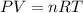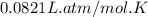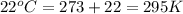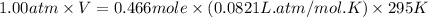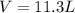Automobile airbags contain solid sodium azide, nan3, that reacts to produce nitrogen gas when heated, thus inflating the bag. 2nan3(s) > 2na(s) + 3n2(g) calculate the value of w (work) for the following system if 20.2 g of nan3 reacts completely at 1.00 atm and 22 degrees

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 17:30
Aroller coaster is traveling at 13 mi./s when you purchase a hill that is 400 m long and down the hill exonerate at 4.0 m/s squared what is the final velocity of the posterior found your answer to the nearest number
Answers: 1

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2
You know the right answer?
Automobile airbags contain solid sodium azide, nan3, that reacts to produce nitrogen gas when heated...
Questions

History, 03.12.2021 23:10

History, 03.12.2021 23:10


Chemistry, 03.12.2021 23:10



Biology, 03.12.2021 23:10

Mathematics, 03.12.2021 23:10


Social Studies, 03.12.2021 23:10

Mathematics, 03.12.2021 23:10






Business, 03.12.2021 23:10



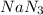
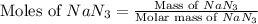
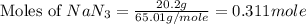
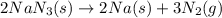
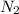
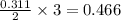 moles of
moles of