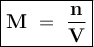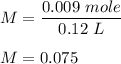In an oxidation-reduction reaction, 0.0450 mol of aqueous feso4 (source of fe2+) reacts completely with 120.0 ml of an acidified aqueous solution of kmno4 (source of 5fe2+(aq) + mno4-(aq) + 8h+(aq) → 5fe3+(aq) + mn2+(aq) + 4h2o(ℓ) what is the molarity of the kmno4 solution?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
What volume of a 2.00 m stock solution of naoh is needed to prepare 150. ml of 0.40 m solution?
Answers: 2

Chemistry, 21.06.2019 23:50
Why do scientists look for patterns in the world? a. patterns can explain observations. b. patterns never change, no matter what. c. patterns are easy for scientists to detect. d. patterns are all the same, through all time.
Answers: 1


Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2
You know the right answer?
In an oxidation-reduction reaction, 0.0450 mol of aqueous feso4 (source of fe2+) reacts completely w...
Questions

Mathematics, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

English, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

English, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Social Studies, 18.09.2020 14:01

English, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Social Studies, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Biology, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Biology, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01