Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2
You know the right answer?
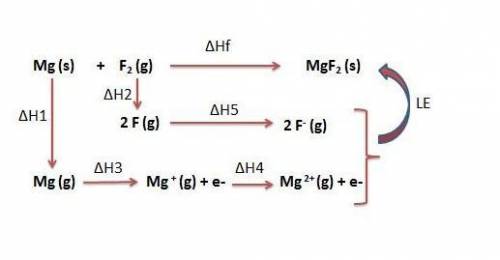
Use the following to calculate h°lattice of mgf2. mg(s) mg(g) h° = 148 kj f2(g) 2 f(g) h° = 159 kj m...
Questions

English, 16.06.2021 14:00



Chemistry, 16.06.2021 14:00

Mathematics, 16.06.2021 14:00



Social Studies, 16.06.2021 14:00

Mathematics, 16.06.2021 14:00



Physics, 16.06.2021 14:00

English, 16.06.2021 14:00

English, 16.06.2021 14:00


History, 16.06.2021 14:00



Computers and Technology, 16.06.2021 14:00

Business, 16.06.2021 14:00