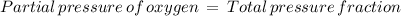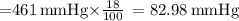Chemistry, 22.11.2019 09:31 nevaehkirk1997
The atmospheric pressure at this altitude is 461 mmhg. assuming that the atmosphere is 18% oxygen (by volume), calculate the partial pressure of o2.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:50
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2

Chemistry, 22.06.2019 00:50
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

You know the right answer?
The atmospheric pressure at this altitude is 461 mmhg. assuming that the atmosphere is 18% oxygen (b...
Questions




Mathematics, 07.12.2021 22:00



Physics, 07.12.2021 22:00

Social Studies, 07.12.2021 22:00



English, 07.12.2021 22:00

Mathematics, 07.12.2021 22:00

Biology, 07.12.2021 22:00




English, 07.12.2021 22:00

Chemistry, 07.12.2021 22:00


Mathematics, 07.12.2021 22:00