Chemistry, 22.11.2019 20:31 alexfvdsdfgfd8151
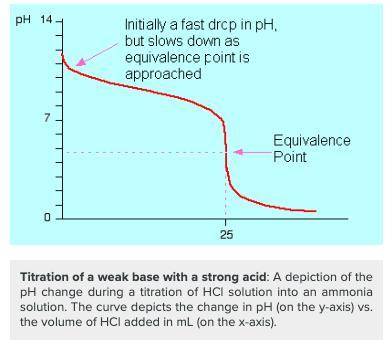
Which of the following would you identify a titration curve that involved a strong acid titrated by a weak base?
a. the ph at the equivalence point is lower than 7.
b. the ph at the equivalence point is higher than 7.
c. the titration curve begins at a higher ph and ends at a lower ph.
d. there is a rapid change in ph near the equivalence point (ph = 7).

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Write the symbol for every chemical element that has atomic number greater than 3 and atomic mass less than 12.0 u.
Answers: 1

Chemistry, 22.06.2019 04:30
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1
You know the right answer?
Which of the following would you identify a titration curve that involved a strong acid titrated by...
Questions

Arts, 07.10.2020 14:01

Mathematics, 07.10.2020 14:01

Mathematics, 07.10.2020 14:01



Mathematics, 07.10.2020 14:01

English, 07.10.2020 14:01

History, 07.10.2020 14:01

History, 07.10.2020 14:01

History, 07.10.2020 14:01

Mathematics, 07.10.2020 14:01


History, 07.10.2020 14:01

Mathematics, 07.10.2020 14:01




History, 07.10.2020 14:01


History, 07.10.2020 14:01