
Chemistry, 04.11.2019 14:31 danielzgame
In an experiment, 12.0 dm3 of oxygen, measured under room conditions, is used to burn completely 0.10 mol of propan-1-ol. what is the final volume of gas, measured under room conditions? a 7.20 dm3 b 8.40 dm3 c 16.8 dm3 d 18.00 dm3

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

Chemistry, 22.06.2019 12:20
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l.s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

You know the right answer?
In an experiment, 12.0 dm3 of oxygen, measured under room conditions, is used to burn completely 0.1...
Questions

History, 12.03.2020 07:03

Mathematics, 12.03.2020 07:03


Mathematics, 12.03.2020 07:03


Mathematics, 12.03.2020 07:03

Mathematics, 12.03.2020 07:03


Mathematics, 12.03.2020 07:03


Mathematics, 12.03.2020 07:03

Mathematics, 12.03.2020 07:03



Mathematics, 12.03.2020 07:03

Biology, 12.03.2020 07:03

Mathematics, 12.03.2020 07:03


Biology, 12.03.2020 07:03

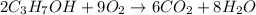
 )
) then, 0.10 moles of propanol will give:
then, 0.10 moles of propanol will give: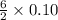 moles of
moles of 





