
Chemistry, 22.11.2019 21:31 samueltaye
Calculate δh and δstot when two copper blocks, each of mass 10.0 kg, one at 100°c and the other at 0°c, are placed in contact in an isolated container. the specific heat capacity of copper is 0.385 j k−1 g−1 and may be assumed constant over the temperature range involved.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1

Chemistry, 22.06.2019 22:30
The diagram shows the relationship between scientific disciplines.the names of some scientific disciplines have been removed from the boxes. which scientific discipline belongs in the blue box? a.physics b.biology c.chemistry d.metallurgy
Answers: 2

Chemistry, 23.06.2019 03:00
The size (radius) of an oxygen molecule is about 2.0 ×10−10m. make a rough estimate of the pressure at which the finite volume of the molecules should cause noticeable deviations from ideal-gas behavior at ordinary temperatures (t= 300k ). assume that deviatons would be noticeable when volume of the gas per molecule equals the volume of the molecule itself.
Answers: 3
You know the right answer?
Calculate δh and δstot when two copper blocks, each of mass 10.0 kg, one at 100°c and the other at 0...
Questions





Spanish, 03.12.2020 18:40

Physics, 03.12.2020 18:40


Physics, 03.12.2020 18:40

Mathematics, 03.12.2020 18:40





Mathematics, 03.12.2020 18:40


Social Studies, 03.12.2020 18:40


English, 03.12.2020 18:40


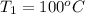 = (100 + 273) K = 373 K
= (100 + 273) K = 373 K 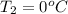 = (0 + 273) K = 273 K
= (0 + 273) K = 273 K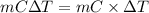
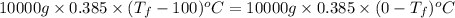
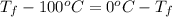
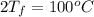
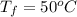
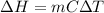
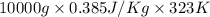
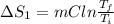
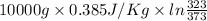
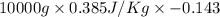
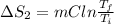
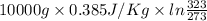
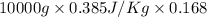
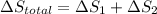
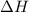 is 1243.5 kJ and
is 1243.5 kJ and 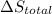 is 93.37 J/K.
is 93.37 J/K.

