
Chemistry, 22.11.2019 22:31 maheshwarlall
The value of δg° at 25 °c for the decomposition of gaseous sulfur trioxide to solid elemental sulfur and gaseous oxygen, 2so3 (g) → 2s (s, rhombic) + 3o2 (g) is kj/mol. the value of g° at 25 °c for the decomposition of gaseous sulfur trioxide to solid elemental sulfur and gaseous oxygen, 2so3 (g) 2s (s, rhombic) + 3o2 (g) is kj/mol. +740.8 -370.4 +185.2 +370.4 -740.8

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Asample of silver (with work function ? = 4.52 ev) is exposed to an ultraviolet light source (? = 200 nm), which results in the ejection of photoelectrons. what changes will be observed if: silver is replaced with copper (? = 5.10 ev) more photoelectrons ejected no photoelectrons are emitted fewer photoelectrons ejected more energetic photoelectrons (on average) less energetic photoelectrons (on average)
Answers: 3

Chemistry, 22.06.2019 01:10
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1
You know the right answer?
The value of δg° at 25 °c for the decomposition of gaseous sulfur trioxide to solid elemental sulfur...
Questions

Biology, 06.03.2021 01:40

English, 06.03.2021 01:40





Mathematics, 06.03.2021 01:40


English, 06.03.2021 01:40

English, 06.03.2021 01:40

Mathematics, 06.03.2021 01:40

Biology, 06.03.2021 01:40

Social Studies, 06.03.2021 01:40

Mathematics, 06.03.2021 01:40

Mathematics, 06.03.2021 01:40


Mathematics, 06.03.2021 01:40


Business, 06.03.2021 01:40

Computers and Technology, 06.03.2021 01:40

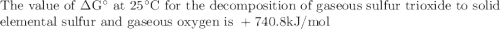


![\begin{array}{l}{\Delta \mathrm{G}^{\circ}=\Sigma \mathrm{G}_{\mathrm{f}(\text { products })}^{\circ}-\Sigma \mathrm{G}_{\text {creatants }}^{\circ}} \\ {\Delta \mathrm{G}^{\circ}=[\mathrm{Sum} \text { of standard free energies of formation of products }]-[\mathrm{Sum}\text { of standard } } \\ {\text { free energies bf formation of reactants] }}\end{array}](/tpl/images/0386/9118/6152f.png)
![\begin{array}{l}{\text { Now here standard values of } \Delta G^{\circ} f(k J / m o l) \text { for } S=0, O_{2}=0 \& S O_{3}=-370.4} \\ {\text { Hence these values can be substituted in above equation: }} \\ {\Delta G^{\circ}=\left[2 G_{f}^{\circ}(0)+3 G_{f}(0)\right]-[2(-370.4)]} \\ {\Delta G^{\circ}=[-0+0]-[-740.8]} \\ {\Delta G^{\circ}=+740.8 \mathrm{kJ} / \mathrm{mol}}\end{array}](/tpl/images/0386/9118/e4e39.png)


