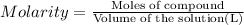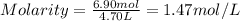Chemistry, 22.11.2019 23:31 shealwaysknows23
An aqueous magnesium chloride solution is made by dissolving 6.90 moles of mgcl 2 in sufficient water so that the final volume of the solution is 4.70 l . calculate the molarity of the mgcl 2 solution.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 3
You know the right answer?
An aqueous magnesium chloride solution is made by dissolving 6.90 moles of mgcl 2 in sufficient wate...
Questions


Mathematics, 26.04.2021 02:50








Mathematics, 26.04.2021 02:50


Mathematics, 26.04.2021 02:50

Mathematics, 26.04.2021 02:50

Mathematics, 26.04.2021 02:50

History, 26.04.2021 02:50

Mathematics, 26.04.2021 02:50



Mathematics, 26.04.2021 02:50

Biology, 26.04.2021 02:50