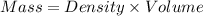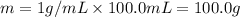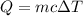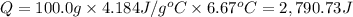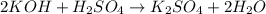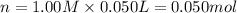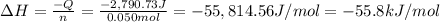When 50.0 ml of 0.500 m h2so4 is added to 50.0 ml of 1.00 m koh in a coffee cup calorimeter, the temperature of the solution rises from 25.10°c to 31.77°c. calculate δh of this reaction (in kj/mol koh). assume that the total volume is the sum of the individual volumes and that the density and specific heat capacity of the solution are the same as that for pure water (c = 4.184j/g°c).a. -112 kj/mol kohb. -2.79 kj/mol kohc. -74.9 kj/mol kohd. -1.86 kj/mol kohe. -55.8 kj/mol koh

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 01:30
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2
You know the right answer?
When 50.0 ml of 0.500 m h2so4 is added to 50.0 ml of 1.00 m koh in a coffee cup calorimeter, the tem...
Questions



Health, 02.07.2020 21:01



Mathematics, 02.07.2020 21:01

Social Studies, 02.07.2020 21:01


Mathematics, 02.07.2020 21:01

Mathematics, 02.07.2020 21:01



Mathematics, 02.07.2020 21:01





Social Studies, 02.07.2020 21:01