
Determine the mole fractions of each component in the vapor phase of the vapor in equilibrium with a 1: 1 molar ratio of hexane (c6h14) and cyclohexane (c6h12). the equilibrium vapor pressures of hexane and cyclohexane are equal to 151.4 torr and 97.6 torr respectively

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3


Chemistry, 22.06.2019 12:20
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l.s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3
You know the right answer?
Determine the mole fractions of each component in the vapor phase of the vapor in equilibrium with a...
Questions


English, 03.09.2021 20:20

Chemistry, 03.09.2021 20:20


History, 03.09.2021 20:20



Chemistry, 03.09.2021 20:20

History, 03.09.2021 20:20

Mathematics, 03.09.2021 20:20

Mathematics, 03.09.2021 20:20



Computers and Technology, 03.09.2021 20:20



History, 03.09.2021 20:20


Mathematics, 03.09.2021 20:20

Mathematics, 03.09.2021 20:20

 and
and 
 = pressure in the pure state
= pressure in the pure state


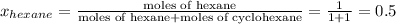 ,
,  ,
, 


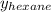 = mole fraction of hexane in vapor phase
= mole fraction of hexane in vapor phase 



