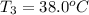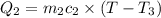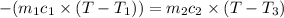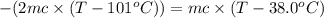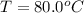Chemistry, 23.11.2019 00:31 sidallen05
One piece of copper jewelry at 101°c has twice the mass of another piece, which is at 38.0°c. both pieces are placed inside a calorimeter of negligible heat capacity. what is the final temperature inside the calorimeter (c of copper=0.387 j/g. k)?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1

Chemistry, 22.06.2019 01:30
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 23.06.2019 00:50
50 points! need answer asap. what type of organic compound contains the following functional group? (2 points)
Answers: 3
You know the right answer?
One piece of copper jewelry at 101°c has twice the mass of another piece, which is at 38.0°c. both p...
Questions




Mathematics, 24.02.2021 19:20


Physics, 24.02.2021 19:20

Chemistry, 24.02.2021 19:20


Mathematics, 24.02.2021 19:20

Mathematics, 24.02.2021 19:20


Mathematics, 24.02.2021 19:20






Medicine, 24.02.2021 19:20

Mathematics, 24.02.2021 19:20

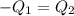
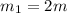
 = 0.387 J/g.K
= 0.387 J/g.K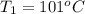
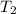 =T
=T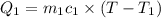
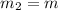
 = 0.387 J/g.K
= 0.387 J/g.K