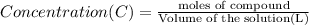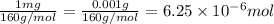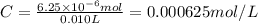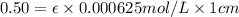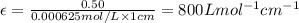Chemistry, 23.11.2019 00:31 chelseychew32
One milligram of a compound of molecular weight 160 is dissolved in 10 ml of ethanol, and the solution is poured into a 1-cm uv cell. the uv spectrum is taken, and there is an absorption at = 247 nm. the maximum absorbance at 247 nm is 0.50. calculate the value of for this absorption.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 22:00
Plz ill give u brainliest which of the following steps is not likely to take place during cellular respiration? a.oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. d. energy is used up.
Answers: 3
You know the right answer?
One milligram of a compound of molecular weight 160 is dissolved in 10 ml of ethanol, and the soluti...
Questions


Mathematics, 24.08.2019 17:30

English, 24.08.2019 17:30

English, 24.08.2019 17:30

English, 24.08.2019 17:30

Business, 24.08.2019 17:30


Mathematics, 24.08.2019 17:30



Computers and Technology, 24.08.2019 17:30

History, 24.08.2019 17:30


Mathematics, 24.08.2019 17:30



Mathematics, 24.08.2019 17:30


Computers and Technology, 24.08.2019 17:30

Arts, 24.08.2019 17:30

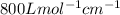 .
.
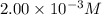
 = molar absorptivity coefficient
= molar absorptivity coefficient