Chemistry, 23.11.2019 02:31 Lawrence101
Gas is trapped inside of a cell with volume 140 m3. the gas exerts 7600 pa of pressure against the walls of the cell. a machine then expands the cell and gives off 490 kj of heat to the gas during the process. if the internal energy changed by 140 kj, what is the final volume of the cell? assume the pressure stays constant.
a. 57 m3
b. 94 m3
c. 660 m3
d. 220 m3
e. 190 m3

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3
You know the right answer?
Gas is trapped inside of a cell with volume 140 m3. the gas exerts 7600 pa of pressure against the w...
Questions














Chemistry, 29.10.2019 00:31



English, 29.10.2019 00:31



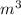 , Pressure = 7600 Pa
, Pressure = 7600 Pa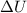 = 140 kJ
= 140 kJ 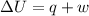
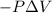
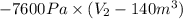
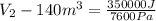
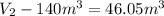
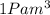 )
)