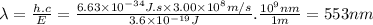The lines on absorption atomic spectra correspond to energies needed for electrons to be excited from a lower energy level to a higher energy level. assume that the energy needed for an electron in 2p orbital in an o atom to jump to 3s orbital is 3.6*10^-19 j, what is its wavelength of the line atomic spectra in nanometer (nm)?
note: use whole numbers and 3 sig figs, or no decimal place.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
**40** points asapessay questions (10 points possible) clear image of next, create your own scenario. it can be one of your own real experiences or one you make up. use imagery in your writing to give your instructor a the setting and an action taking pace in your writing explain the structure and functions of the skin at work in your scenario. !
Answers: 3

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1

Chemistry, 23.06.2019 12:30
Choose one literary selection from this semester in which you think the setting has a great impact on the work. in a full paragraph name the work, describe the setting, and explain why it is so important to the overall story or poem.
Answers: 1

Chemistry, 23.06.2019 12:40
Metric temperature is measured in celsius and fahrenheit. true or false
Answers: 2
You know the right answer?
The lines on absorption atomic spectra correspond to energies needed for electrons to be excited fro...
Questions


Social Studies, 28.04.2021 22:20

Chemistry, 28.04.2021 22:20


Mathematics, 28.04.2021 22:20










Mathematics, 28.04.2021 22:20