
For a beaker containing the reaction below that is at equilibrium, using lechatlier's principle, predict which way the equilibrium conditions will shift (type in left, right, or no change) if silver chloride was added to a beaker.
agno3 (aq) + nacl (aq) → agcl (s) + nano3 (aq)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2

Chemistry, 23.06.2019 07:00
The following transition occurs at a molecular level for a substance. what transition corresponds to this change in microscopic structure? the carbon dioxide molecules on the left are in a regular, tightly packed pattern. after heating, it becomes much lower density. a. melting b. boiling c. sublimation d. freezing
Answers: 1
You know the right answer?
For a beaker containing the reaction below that is at equilibrium, using lechatlier's principle, pre...
Questions


English, 26.03.2020 20:52




Spanish, 26.03.2020 20:53




History, 26.03.2020 20:53

Mathematics, 26.03.2020 20:53

Mathematics, 26.03.2020 20:53







Biology, 26.03.2020 20:53

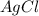 is added.
is added.

