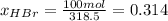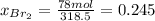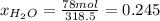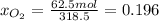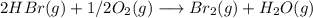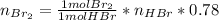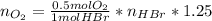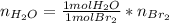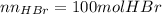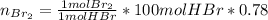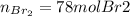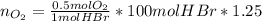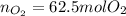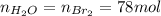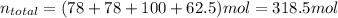Chemistry, 23.11.2019 04:31 rodrickahammonds
Bromine (br2) is produced by reacting hbr with o2, with water as a byproduct. the o2 is part of an air (21 mol % o2, 79 mol % n2) feed stream that is flowing sufficiently fast to provide 25% excess oxygen ("excess" has a precise meaning in process analysis: in this case there is 25% more oxygen than the amount needed to completely react with the limiting reactant). the fractional conversion of hbr is 78%.
a) show the degree of freedom analysis. be as specific as possible about labeling the unknowns and completely write out all of the independent equations.
b) calculate the composition (mole fractions) of the product stream.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:20
What would you do if you told the guy you liked that you liked him
Answers: 1

Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1

You know the right answer?
Bromine (br2) is produced by reacting hbr with o2, with water as a byproduct. the o2 is part of an a...
Questions

English, 19.02.2021 22:50

Mathematics, 19.02.2021 22:50



Mathematics, 19.02.2021 22:50

Mathematics, 19.02.2021 22:50

Mathematics, 19.02.2021 22:50



History, 19.02.2021 22:50


Advanced Placement (AP), 19.02.2021 22:50

Spanish, 19.02.2021 22:50

History, 19.02.2021 22:50


Mathematics, 19.02.2021 22:50

History, 19.02.2021 22:50



History, 19.02.2021 22:50