
Naoh(s) dissolves readily in water to form na+(aq) and oh-(aq). if 35.1 g of naoh is dissolved in 199 g of water in a coffee cup calorimeter, the temperature of the resulting solution increases from 23.0 oc to 62.6 oc. calculate the enthalpy change per mole of naoh dissolved. assume the specific heat capacity of the solution is 4.18 j/oc*g.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation. 8. when a 2.5 mol of sugar (c12h22o11) are added to a certain amount of water the boiling point is raised by 1 celsius degree. if 2.5 mol of aluminum nitrate is added to the same amount of water, by how much will the boiling point be changed? show all calculations leading to your answer or use 3 – 4 sentences to explain your answer. 9. if 5.40 kcal of heat is added to 1.00 kg of water at 100⁰c, how much steam at 100⁰c is produced? show all calculations leading to an answer. 10. the freezing of water at 0⁰c can be represented as follows: h2o (l) ↔ h2o(s) the density of liquid water is 1.00 g/cm3. the density of ice is 0.92 g/cm3. in 3 – 4 sentences explain why applying pressure causes ice to melt.
Answers: 1

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

You know the right answer?
Naoh(s) dissolves readily in water to form na+(aq) and oh-(aq). if 35.1 g of naoh is dissolved in 19...
Questions




Social Studies, 11.11.2020 21:00

Business, 11.11.2020 21:00



Mathematics, 11.11.2020 21:00

Biology, 11.11.2020 21:00


Mathematics, 11.11.2020 21:00

Health, 11.11.2020 21:00


Chemistry, 11.11.2020 21:00




Mathematics, 11.11.2020 21:00

Mathematics, 11.11.2020 21:00

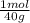 = 0,878 moles
= 0,878 moles

