
Chemistry, 23.11.2019 05:31 lazavionadams81
Find an expression for the change in entropy when two blocks of the same substance of equal mass, one at the temperature th and the other at tc, are brought into contact and allowed to reach equilibrium. evaluate the change for the two blocks of copper, each of mass 500 grams with cpcm= 24.4 j kt-1 mol-1, taking th = 500 k and tc = 250 k.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Each of the following compounds contains a metal that can exhibit more than one ionic charge. provide systematic names for each of these compounds. (a) cr(clo3)6 (b) mo(cn)6 (c) cr2(so3)3 (d) v(clo2)2 (e) v(cn)5 (f) os(clo2)4
Answers: 3

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2
You know the right answer?
Find an expression for the change in entropy when two blocks of the same substance of equal mass, on...
Questions







Mathematics, 07.09.2021 01:00



Mathematics, 07.09.2021 01:00



Mathematics, 07.09.2021 01:00




Chemistry, 07.09.2021 01:00



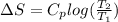
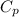 = 24.4 J/mol K
= 24.4 J/mol K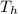 = 500 K,
= 500 K, 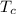 = 250 K
= 250 K 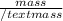
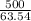
![7.86 \times 24.4 \times [T_{f} - 250]](/tpl/images/0387/6495/f5240.png) =
= ![7.86 \times 24.4 \times [500 -T_{f}]](/tpl/images/0387/6495/72b18.png)
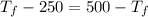
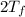 = 750
= 750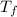 =
= 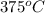
![24.4 log [\frac{375}{500}]](/tpl/images/0387/6495/8e24d.png)
![24.4 log [\frac{375}{250}]](/tpl/images/0387/6495/56e73.png)


