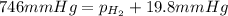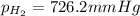Chemistry, 23.11.2019 05:31 Simplytaylorgrenade
Areaction of 0.028 g of magnesium with excess hydrochloric acid generated 31.0 ml of hydrogen gas. the gas was collected by water displacement in a 22 °c water bath. the barometric pressure in the lab that day was 746 mm hg. use dalton's law to calculate the partial pressure of hydrogen gas in the gas-collecting tube.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 00:20
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1


Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1
You know the right answer?
Areaction of 0.028 g of magnesium with excess hydrochloric acid generated 31.0 ml of hydrogen gas. t...
Questions



Business, 19.12.2019 17:31



Geography, 19.12.2019 17:31

Spanish, 19.12.2019 17:31



Mathematics, 19.12.2019 17:31


English, 19.12.2019 17:31

History, 19.12.2019 17:31

Mathematics, 19.12.2019 17:31


Business, 19.12.2019 17:31




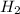 is, 726.2 mmHg
is, 726.2 mmHg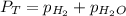
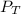 = total partial pressure = barometric pressure = 746 mmHg
= total partial pressure = barometric pressure = 746 mmHg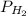 = partial pressure of hydrogen gas = ?
= partial pressure of hydrogen gas = ?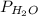 = partial pressure of water vapor = 19.8 mmHg (assume)
= partial pressure of water vapor = 19.8 mmHg (assume)