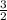Chemistry, 23.11.2019 21:31 michelle453
For the reaction, 2a + b → 3c, rate of disappearance of a is 0.3 m/s in a certain time interval. what is the average rate of appearance of c and what is the average rate of the reaction in that time interval?
a) rate of appearance of c = 0.45 m/s
rate of the reaction = 0.6 m/s
b) rate of appearance of c = 0.2 m/s
rate of the reaction = 0.6 m/s
c) rate of appearance of c = 0.2 m/s
rate of the reaction = 0.15 m/s
d) rate of appearance of c = 0.45 m/s
rate of the reaction = 0.3 m/s
e) rate of appearance of c = 0.45 m/s
rate of the reaction = 0.15 m/s

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 3

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3
You know the right answer?
For the reaction, 2a + b → 3c, rate of disappearance of a is 0.3 m/s in a certain time interval. wha...
Questions

Biology, 26.08.2021 03:40

Mathematics, 26.08.2021 03:40

Biology, 26.08.2021 03:40

History, 26.08.2021 03:40

Mathematics, 26.08.2021 03:40




History, 26.08.2021 03:40


Mathematics, 26.08.2021 03:40

Biology, 26.08.2021 03:40

Mathematics, 26.08.2021 03:40

Mathematics, 26.08.2021 03:40




Mathematics, 26.08.2021 03:40


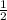
![\frac{d[A]}{dt}](/tpl/images/0388/2473/59baa.png) = -
= -![\frac{d[B]}{dt}](/tpl/images/0388/2473/a0d46.png) =
= 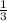
![\frac{d[C]}{dt}](/tpl/images/0388/2473/484ab.png) → equation 1
→ equation 1