Consider the following reversible reaction.
what is the equilibrium constant expression...

Chemistry, 24.11.2019 08:31 vapelordcarl69
Consider the following reversible reaction.
what is the equilibrium constant expression for the given system?
1st pic is equation, 2nd is a, 3rd is b, 4th is c, and 5th is d.
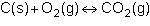
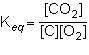
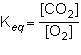
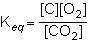
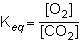

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:00
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2

Chemistry, 23.06.2019 00:30
Maya wrote if you step to describe how carbon circulates between the atmosphere and living organisms
Answers: 1

Chemistry, 23.06.2019 00:30
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1
You know the right answer?
Questions



Health, 25.06.2019 09:10


Spanish, 25.06.2019 09:10









Mathematics, 25.06.2019 09:10


Mathematics, 25.06.2019 09:10






