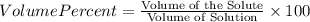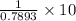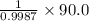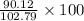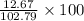Chemistry, 24.11.2019 12:31 Samanthas6365
Find both the volume percent of a solution that has 10.0 g of ethanol (d = 0.7893 g/ml) and 90.0 g of water (d = 0.9987 g/ml). assume volumes are additive.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
If you have 5.25 grams of methane (ch4), how many grams of co2 will you produce ?
Answers: 1

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1
You know the right answer?
Find both the volume percent of a solution that has 10.0 g of ethanol (d = 0.7893 g/ml) and 90.0 g o...
Questions

Mathematics, 12.04.2021 09:00

Mathematics, 12.04.2021 09:00

Mathematics, 12.04.2021 09:00


English, 12.04.2021 09:00

Mathematics, 12.04.2021 09:00

World Languages, 12.04.2021 09:00

Biology, 12.04.2021 09:00

History, 12.04.2021 09:00


World Languages, 12.04.2021 09:00



Geography, 12.04.2021 09:00

Biology, 12.04.2021 09:00

Mathematics, 12.04.2021 09:00



History, 12.04.2021 09:00

Mathematics, 12.04.2021 09:00