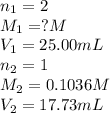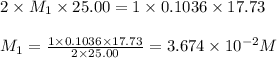Chemistry, 25.11.2019 19:31 michellectucker1982
Astudent titrated a 25.00-ml sample of a solution containing an unknown weak, diprotic acid (h2a) with n2oh. if the titration required 17.73 ml of 0.1036 m n2oh to completely neutralize the acid, calculate the concentration (in m) of the weak acid in the sample.
(a) 9.184 x 10 m
(b) 3.674 x 10-2 m
(c) 7.304 x 10-2 m
(d) 7.347 x 10-2 m
(e) 1.469 x 101 m

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

Chemistry, 23.06.2019 01:30
How does the attraction between particles affect the ability of a solvent to dissolve in a substance
Answers: 1
You know the right answer?
Astudent titrated a 25.00-ml sample of a solution containing an unknown weak, diprotic acid (h2a) wi...
Questions


Mathematics, 18.03.2021 19:50

Chemistry, 18.03.2021 19:50

Biology, 18.03.2021 19:50

Geography, 18.03.2021 19:50

History, 18.03.2021 19:50



English, 18.03.2021 19:50

Mathematics, 18.03.2021 19:50

Mathematics, 18.03.2021 19:50


Biology, 18.03.2021 19:50



Mathematics, 18.03.2021 19:50

English, 18.03.2021 19:50

Biology, 18.03.2021 19:50

Geography, 18.03.2021 19:50

Mathematics, 18.03.2021 19:50

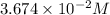
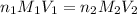
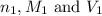 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 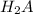
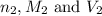 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.