
Chemistry, 25.11.2019 20:31 jakobrobinette
Kemmi major does some experimental work on the combustion of sucrose: c12h22o11(s) 12 o2(g) → 12 co2(g) 11 h2o(g) she burns a 0.05392 g pellet of sucrose in a bomb calorimeter with excess oxygen. she determines the qrxn to be –916.6 j for the reaction. calculate the ∆h value for the combustion reaction. (round the answer to 3 significant digits, units of kj, pay attention to positive or negative.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1

Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1


Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3
You know the right answer?
Kemmi major does some experimental work on the combustion of sucrose: c12h22o11(s) 12 o2(g) → 12 co...
Questions


Mathematics, 27.02.2020 20:48

Mathematics, 27.02.2020 20:48

Health, 27.02.2020 20:48

Mathematics, 27.02.2020 20:48






Computers and Technology, 27.02.2020 20:48




Health, 27.02.2020 20:48




Mathematics, 27.02.2020 20:48

Physics, 27.02.2020 20:48




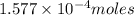 of sucrose releases = 916.6 J of heat
of sucrose releases = 916.6 J of heat
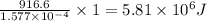 of heat
of heat


