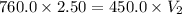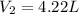Chemistry, 25.11.2019 20:31 bloodyflower2003
The manufacturer specs for a particular balloon indicate the maximum inflated volume is 3.00 l. the balloon is filled with 2.50 l of helium at sea level (assume p= 1.00 atm) and released. when the balloon rises to a higher altitude where the pressure is 450.0 mm hg, will the balloon burst? show a calculation to support your answer. assume constant temperature.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1
You know the right answer?
The manufacturer specs for a particular balloon indicate the maximum inflated volume is 3.00 l. the...
Questions



English, 22.10.2020 01:01


Arts, 22.10.2020 01:01





English, 22.10.2020 01:01

French, 22.10.2020 01:01

Mathematics, 22.10.2020 01:01



Health, 22.10.2020 01:01


Mathematics, 22.10.2020 01:01


Business, 22.10.2020 01:01

Chemistry, 22.10.2020 01:01

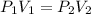
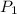 = initial pressure of gas= 1.00 atm = 760.0 mm Hg
= initial pressure of gas= 1.00 atm = 760.0 mm Hg 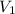 = initial volume of gas = 2.50 L
= initial volume of gas = 2.50 L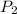 = final pressure of gas= 450.0 mm Hg
= final pressure of gas= 450.0 mm Hg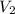 = final volume of gas = ?
= final volume of gas = ?