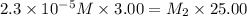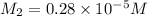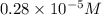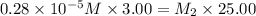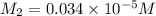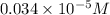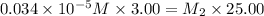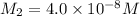Chemistry, 25.11.2019 20:31 monicaharris3
Suppose you start with a solution of red dye #40 that is 2.3 ✕ 10−5 m. if you do three successive volumetric dilutions pipetting 3.00 ml of solution and diluting with water in a 25.00 ml volumetric flask, what is the molarity of the final dilution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1

Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

You know the right answer?
Suppose you start with a solution of red dye #40 that is 2.3 ✕ 10−5 m. if you do three successive vo...
Questions

Mathematics, 19.07.2019 03:00


Mathematics, 19.07.2019 03:00

History, 19.07.2019 03:00


Arts, 19.07.2019 03:00

SAT, 19.07.2019 03:00


History, 19.07.2019 03:00







Mathematics, 19.07.2019 03:00




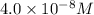
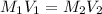
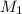 = molarity of stock solution =
= molarity of stock solution = 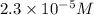
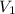 = volume of stock solution = 3.00 ml
= volume of stock solution = 3.00 ml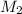 = molarity of diluted solution = ?
= molarity of diluted solution = ?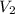 = volume of diluted solution = 25.00 ml
= volume of diluted solution = 25.00 ml