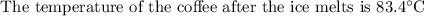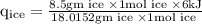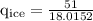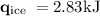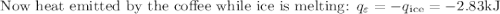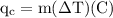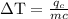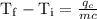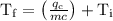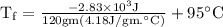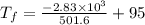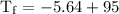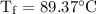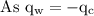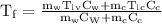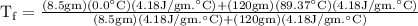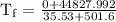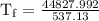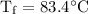Chemistry, 25.11.2019 21:31 jenorajordan5387
An ice cube of mass 8.5g is added to a cup of coffee, whose temperature is 95 degrees celcius and which contains 120 g of liquid. assume the specific heat capacity of coffee is the same as that of water. the heat of fusion of the ice (the heat associated with ice melting) is 6.0 kj/mol. find the temperature of the coffee after the ice melts.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2


Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3

Chemistry, 23.06.2019 13:30
Malik formed a hypothesis that an increase in atmospheric oxygen levels by 10% would cause red-legged grasshoppers to grow larger than normal. suppose that malik performs an experiment to test his hypothesis. which of these actions would represent a scientific mistake in his experiment? a. he experiments on live grasshoppers instead of preserved ones. b. he focuses on red-legged grasshoppers instead of all kinds of grasshoppers. c. he varies the nitrogen and carbon dioxide levels in the air from one trial to the next. d. he conducts the experiment in a controlled lab setting with a lab partner. e. he measures the mass and length of his specimens at the start of each trial.
Answers: 1
You know the right answer?
An ice cube of mass 8.5g is added to a cup of coffee, whose temperature is 95 degrees celcius and wh...
Questions



Spanish, 08.09.2021 03:30

History, 08.09.2021 03:30





Mathematics, 08.09.2021 03:30

Mathematics, 08.09.2021 03:30


English, 08.09.2021 03:30

Physics, 08.09.2021 03:30


Mathematics, 08.09.2021 03:30




Mathematics, 08.09.2021 03:30