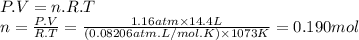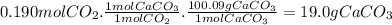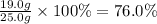Chemistry, 25.11.2019 21:31 zanaplen27
For the reaction below, kp = 1.16 at 800.°c. caco3(s) equilibrium reaction arrow cao(s) + co2(g) if a 25.0-g sample of caco3 is put into a 14.4 l container and heated to 800°c, what percentage by mass of the caco3 will react to reach equilibrium?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 23.06.2019 00:00
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1
You know the right answer?
For the reaction below, kp = 1.16 at 800.°c. caco3(s) equilibrium reaction arrow cao(s) + co2(g) if...
Questions





Mathematics, 17.12.2020 19:10

Spanish, 17.12.2020 19:10

Mathematics, 17.12.2020 19:10




Mathematics, 17.12.2020 19:10




Mathematics, 17.12.2020 19:10


Advanced Placement (AP), 17.12.2020 19:20

Chemistry, 17.12.2020 19:20


Mathematics, 17.12.2020 19:20