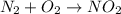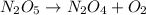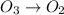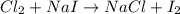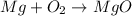Be sure to answer all parts. write an unbalanced equation to represent each of the following reactions: do not include phase abbreviations. (a) nitrogen and oxygen react to form nitrogen dioxide. (b) dinitrogen pentoxide reacts to form dinitrogen tetroxide and oxygen. (c) ozone reacts to form oxygen. (d) chlorine and sodium iodide react to form iodine and sodium chloride. (e) magnesium and oxygen react to form magnesium oxide.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1
You know the right answer?
Be sure to answer all parts. write an unbalanced equation to represent each of the following reactio...
Questions

Mathematics, 06.03.2020 14:03

Mathematics, 06.03.2020 14:03






Mathematics, 06.03.2020 14:03


Mathematics, 06.03.2020 14:04



Spanish, 06.03.2020 14:04



Mathematics, 06.03.2020 14:05


Biology, 06.03.2020 14:05

Health, 06.03.2020 14:05

Mathematics, 06.03.2020 14:05