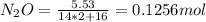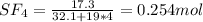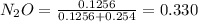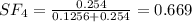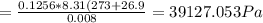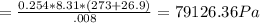A8.00 l tank at 26.9 c is filled with 5.53 g of dinitrogen difluoride gas and 17.3 g of sulfur hexafluoride gas. you can assume both gases behave as ideal gases under these conditions. calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:40
Modern pennies are composed of zinc coated with copper. a student determines the mass of a penny to be 2.482 g and then makes several scratches in the copper coaling (to expose the underlying zinc). the student puts the scratched penny in hydrochloric acid, where the following reaction occurs between the zinc and the hcl (the copper remains undissolved): zn(s) + 2 hcl(aq) → h2(g) + zncl(aq)the student collects the hydrogen produced over water at 25 °c. the collected gas occupies a volume of 0.899 l at a total pressure of 79 j mmhg. calculate the percent zinc (by mass) in the penny. (assume that all the zn in the penny dissolves.)
Answers: 1


Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1
You know the right answer?
A8.00 l tank at 26.9 c is filled with 5.53 g of dinitrogen difluoride gas and 17.3 g of sulfur hexaf...
Questions


Mathematics, 24.06.2021 17:40


Mathematics, 24.06.2021 17:40

Mathematics, 24.06.2021 17:40


Physics, 24.06.2021 17:40


Mathematics, 24.06.2021 17:40







Mathematics, 24.06.2021 17:40

Mathematics, 24.06.2021 17:40


Mathematics, 24.06.2021 17:40