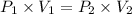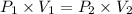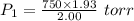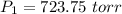Chemistry, 25.11.2019 22:31 adantrujillo1234
A2.00-l sample of was collected over water at a total pressure of 750. torr and 26 °c. when the was dried (water vapor removed), the gas had a volume of 1.93 l at 26 °c and 750. torr. calculate the vapor pressure of water at 26 °c.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2

Chemistry, 22.06.2019 20:00
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1


Chemistry, 22.06.2019 20:30
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1
You know the right answer?
A2.00-l sample of was collected over water at a total pressure of 750. torr and 26 °c. when the was...
Questions

Mathematics, 25.01.2021 21:50

Mathematics, 25.01.2021 21:50

Mathematics, 25.01.2021 21:50

Chemistry, 25.01.2021 21:50





Biology, 25.01.2021 21:50

Mathematics, 25.01.2021 21:50

Mathematics, 25.01.2021 21:50

Physics, 25.01.2021 21:50

English, 25.01.2021 21:50

Mathematics, 25.01.2021 21:50





Mathematics, 25.01.2021 21:50