Chemistry, 25.11.2019 23:31 ZeroFrost7899
Calculate δh∘f (in kilojoules per mole) for benzene, c6h6, from the following data: 2c6h6(l) + 15o2(g)→12co2(g)+6h2o(l) δh∘=−6534.0 kjδh∘f co2=−393.5 kj/molδh∘f h2o=−285.8 kj/mol express the enthalpy change in kilojoules per mole to three significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
The diagram shows the structures of horse and cat forelimbs. what does the diagram suggest about the evolutionary relationship between these two mammals? a. they have homologous structures, indicating a common ancestor. b. they have analogous structures, indicating a common ancestor. c. they have homologous structures, indicating that they do not have a common ancestor. d. they have analogous structures, indicating that they do not have a common ancestor.
Answers: 2

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 12:10
|using the periodic tablewarm-upuse the periodic table in the tools bar to answer the following questions.what elemental classification does oxygen belongto? done
Answers: 3
You know the right answer?
Calculate δh∘f (in kilojoules per mole) for benzene, c6h6, from the following data: 2c6h6(l) + 15o2...
Questions





English, 30.01.2020 10:57

Mathematics, 30.01.2020 10:57

English, 30.01.2020 10:57




Mathematics, 30.01.2020 10:57


Mathematics, 30.01.2020 10:57

Computers and Technology, 30.01.2020 10:57


History, 30.01.2020 10:57


English, 30.01.2020 10:57


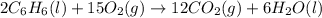
![\Delta H=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/0390/4702/76c37.png)
![\Delta H=[(n_{CO_2}\times \Delta H_{CO_2})+(n_{H_2O}\times \Delta H_{H_2O})]-[(n_{O_2}\times \Delta H_{O_2})+(n_{C_6H_6}\times \Delta H_{C_6H_6})]](/tpl/images/0390/4702/15aa8.png)
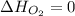 (as heat of formation of substances in their standard state is zero
(as heat of formation of substances in their standard state is zero![-6534.0=[(12\times -393.5)+(6\times -285.8)]-[(15\times 0)+(2\times \Delta H_{C_6H_6})]](/tpl/images/0390/4702/ed6d2.png)