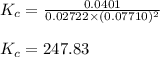Chemistry, 26.11.2019 00:31 bella122805
Amixture of gaseous co and h2, called synthesis gas, is used commercially to prepare methanol (ch3oh), a compound considered an alternative fuel to gasoline. under equilibrium conditions at 550.3 k, [h2] = 0.07710 mol/l, [co] = 0.02722 mol/l, and [ch3oh] = 0.0401 mol/l. what is the value of kc for this reaction at 550.3 k?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:50
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2

Chemistry, 22.06.2019 00:30
Drive down any three characteristic of modern periodic table
Answers: 1

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2
You know the right answer?
Amixture of gaseous co and h2, called synthesis gas, is used commercially to prepare methanol (ch3oh...
Questions

Mathematics, 10.12.2020 22:50

Chemistry, 10.12.2020 22:50

English, 10.12.2020 22:50

Mathematics, 10.12.2020 22:50


Mathematics, 10.12.2020 22:50

Mathematics, 10.12.2020 22:50

Chemistry, 10.12.2020 22:50

Mathematics, 10.12.2020 22:50


Computers and Technology, 10.12.2020 22:50


Mathematics, 10.12.2020 22:50

History, 10.12.2020 22:50

Mathematics, 10.12.2020 22:50

English, 10.12.2020 22:50

Mathematics, 10.12.2020 22:50

Biology, 10.12.2020 22:50


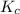 for the reaction at 550.3 K is 247.83
for the reaction at 550.3 K is 247.83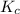
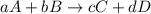
![K_{c}=\frac{[C]^c[D]^d}{[A]^a[B]^b}](/tpl/images/0390/5865/b6f47.png)
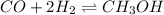
![K_c=\frac{[CH_3OH]}{[CO][H_2]^2}](/tpl/images/0390/5865/4cf94.png)
![[CH_3OH]=0.0401mol/L](/tpl/images/0390/5865/4486b.png)
![[CO]=0.02722mol/L](/tpl/images/0390/5865/ab367.png)
![[H_2]=0.07710mol/L](/tpl/images/0390/5865/326e6.png)