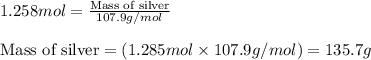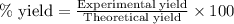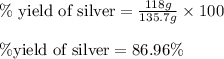Chemistry, 26.11.2019 00:31 agarcia24101993
When 40.0 g of copper are reacted with silver nitrate solution cu + 2 agno3 --> cu(no3)2 + 2 ag 118 g of silver are obtained. what is the percent yield of silver? molar mass of silver = 107.9 g, molar mass of copper = 63.55 g

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Two atoms interact with each other as shown by the equation. complete the equation by filling in the missing parts. 1 2 3 4 5 h he li
Answers: 2

Chemistry, 22.06.2019 04:30
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1

Chemistry, 23.06.2019 04:31
2ki + pb(no3)2 → 2kno3 + pbi2 determine how many moles of kno3 are created if 0.03 moles of ki are completely consumed.
Answers: 1
You know the right answer?
When 40.0 g of copper are reacted with silver nitrate solution cu + 2 agno3 --> cu(no3)2 + 2 ag...
Questions

History, 19.11.2020 01:00


Chemistry, 19.11.2020 01:00

Mathematics, 19.11.2020 01:00


Geography, 19.11.2020 01:00

Biology, 19.11.2020 01:00

Mathematics, 19.11.2020 01:00

Chemistry, 19.11.2020 01:00



Biology, 19.11.2020 01:00


Chemistry, 19.11.2020 01:00

English, 19.11.2020 01:00

History, 19.11.2020 01:00


Physics, 19.11.2020 01:00

English, 19.11.2020 01:00

Mathematics, 19.11.2020 01:00

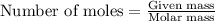 .....(1)
.....(1)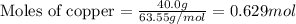
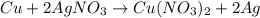
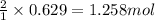 of silver
of silver