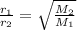Chemistry, 26.11.2019 01:31 jeovontamarley
The rate of effusion of a gas, r, is inversely proportional to the square root of its molar mass, m. the relative rate of two different gases is expressed as r1r2=m2m1−−−√ where r1 and r2 are the effusion rates of two gases and m1 and m2 are their respective molar masses. part a in an effusion experiment, it was determined that nitrogen gas, n2, effused at a rate 1.812 times faster than an unknown gas. what is the molar mass of the unknown gas? express your answer to four significant figures and include the appropriate units.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1
You know the right answer?
The rate of effusion of a gas, r, is inversely proportional to the square root of its molar mass, m....
Questions



Spanish, 13.10.2020 02:01


Physics, 13.10.2020 02:01

World Languages, 13.10.2020 02:01

Mathematics, 13.10.2020 02:01


Biology, 13.10.2020 02:01

Business, 13.10.2020 02:01


Computers and Technology, 13.10.2020 02:01

Mathematics, 13.10.2020 02:01



Mathematics, 13.10.2020 02:01

Mathematics, 13.10.2020 02:01


Mathematics, 13.10.2020 02:01