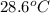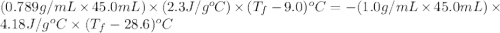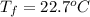Chemistry, 26.11.2019 02:31 kylemartinez13
If 45.0 ml of ethanol (density=0.789 g/ml) initially at 9.0 c is mixed with 45.0 ml of water (density=1.0 g/ml) initially at 28.6 c in an insulated beaker, and assuming that no heat is lost, what is the final temperature of the mixture?
i tried many times in trying to get the answer, but i keep getting it wrong. i appreciate who answers this writes it step by step. you.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:40
You may expect bonds between two atoms which each have n covalent lonic metallic hydrogen
Answers: 2

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

You know the right answer?
If 45.0 ml of ethanol (density=0.789 g/ml) initially at 9.0 c is mixed with 45.0 ml of water (densit...
Questions

Biology, 17.07.2020 02:01


Mathematics, 17.07.2020 02:01


Biology, 17.07.2020 02:01

Mathematics, 17.07.2020 02:01



Mathematics, 17.07.2020 02:01

Mathematics, 17.07.2020 02:01


Mathematics, 17.07.2020 02:01

Mathematics, 17.07.2020 02:01



Mathematics, 17.07.2020 02:01

Mathematics, 17.07.2020 02:01

Mathematics, 17.07.2020 02:01

Mathematics, 17.07.2020 02:01

Mathematics, 17.07.2020 02:01

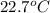
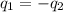
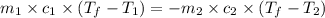
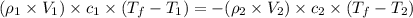
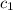 = specific heat of ethanol =
= specific heat of ethanol = 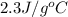
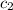 = specific heat of water =
= specific heat of water = 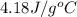
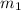 = mass of ethanol
= mass of ethanol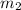 = mass of water
= mass of water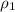 = density of ethanol = 0.789 g/mL
= density of ethanol = 0.789 g/mL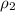 = density of water = 1.0 g/mL
= density of water = 1.0 g/mL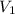 = volume of ethanol = 45.0 mL
= volume of ethanol = 45.0 mL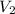 = volume of water = 45.0 mL
= volume of water = 45.0 mL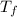 = final temperature of mixture = ?
= final temperature of mixture = ? = initial temperature of ethanol =
= initial temperature of ethanol = 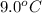
 = initial temperature of water =
= initial temperature of water =