
Chemistry, 26.11.2019 03:31 aghamuzahirali5289
Aphosphate buffer solution (25.00 ml sample) used for a growth medium was titrated with 0.1000 m hydrochloric acid. the components of the buffer were sodium monohydrogenphosphate and sodium dihydrogenphosphate. the first endpoint occurred at a volume of 10.32 ml, and the second occurred after an additional 18.62 ml was added, for a total volume of 28.94 ml. what was the total concentration of phosphate (in any form) in the buffer?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Which substance absorbs 58.16 kj of energy when 3.11 mol vaporizes? a)ch4 b)h2s c)co2 d)nacl
Answers: 2



Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3
You know the right answer?
Aphosphate buffer solution (25.00 ml sample) used for a growth medium was titrated with 0.1000 m hyd...
Questions


English, 23.12.2019 04:31


Computers and Technology, 23.12.2019 04:31





Mathematics, 23.12.2019 04:31

Mathematics, 23.12.2019 04:31

Mathematics, 23.12.2019 04:31


Social Studies, 23.12.2019 04:31

Mathematics, 23.12.2019 04:31

Physics, 23.12.2019 04:31


Mathematics, 23.12.2019 04:31

History, 23.12.2019 04:31


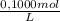 = 1,862x10⁻³moles of HCl ≡ moles of phosphate buffer.
= 1,862x10⁻³moles of HCl ≡ moles of phosphate buffer.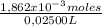 = 0,07448M of phosphate buffer
= 0,07448M of phosphate buffer

