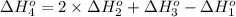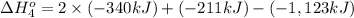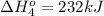Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 23.06.2019 00:00
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1
You know the right answer?
Calculate the enthalpy for this reaction: 2c(s) + h2(g) > c2h2(g) δh° = kj given the following...
Questions

Mathematics, 01.07.2019 10:30

Mathematics, 01.07.2019 10:30


History, 01.07.2019 10:30




Mathematics, 01.07.2019 10:30

History, 01.07.2019 10:30

Health, 01.07.2019 10:30

History, 01.07.2019 10:30

Mathematics, 01.07.2019 10:30

Mathematics, 01.07.2019 10:30

Mathematics, 01.07.2019 10:30

Mathematics, 01.07.2019 10:30

Mathematics, 01.07.2019 10:30

Mathematics, 01.07.2019 10:30


Arts, 01.07.2019 10:30

Mathematics, 01.07.2019 10:30

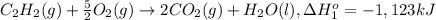 ...[1]
...[1]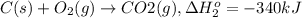 ..[2]
..[2]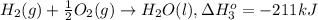 ..[3]
..[3]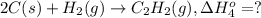 ..[4]
..[4]