Chemistry, 26.11.2019 03:31 ashtonrieper1132
Liquid octane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . suppose 17. g of octane is mixed with 93.0 g of oxygen. calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. be sure your answer has the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1

Chemistry, 22.06.2019 23:00
In the reaction h2co3 (aq) + 3nh3 (aq) = 2 nh4+ (aq) + co3 2-, how many electrons are transferred?
Answers: 3

Chemistry, 23.06.2019 02:00
Anitrogen atom and an oxygen atom combine chemically to form nitric oxide. what is nitric oxide?
Answers: 1

Chemistry, 23.06.2019 05:30
Calculate the temperature rise when 0.2g of propane is used to heat 400cm cubed of water.
Answers: 3
You know the right answer?
Liquid octane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . s...
Questions


English, 21.09.2019 16:10


Health, 21.09.2019 16:10



Mathematics, 21.09.2019 16:10

Mathematics, 21.09.2019 16:10





Mathematics, 21.09.2019 16:20

Computers and Technology, 21.09.2019 16:20

Spanish, 21.09.2019 16:20

Mathematics, 21.09.2019 16:20

Biology, 21.09.2019 16:20

Health, 21.09.2019 16:20


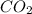
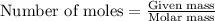
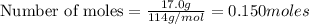
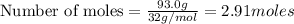
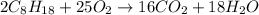
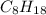 require 25 moles of
require 25 moles of 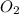
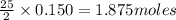 of
of 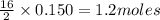 of
of