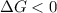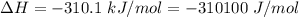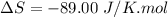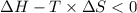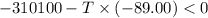Chemistry, 26.11.2019 03:31 Rileyb101207
Areaction will be spontaneous only at low temperatures if both δh and δs are negative. for a reaction in which δh = −310.1 kj/mol and δs = −89.00 j/k · mol, determine the temperature (in °c) below which the reaction is spontaneous.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:10
Starch and are common polysaccharide carbohydrates found in plants. sucrose glycogen fructose cellulose
Answers: 3

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1


Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2
You know the right answer?
Areaction will be spontaneous only at low temperatures if both δh and δs are negative. for a reactio...
Questions


Mathematics, 15.04.2021 20:00

Mathematics, 15.04.2021 20:00

Mathematics, 15.04.2021 20:00

Mathematics, 15.04.2021 20:00

Mathematics, 15.04.2021 20:00


Mathematics, 15.04.2021 20:00



Mathematics, 15.04.2021 20:00

Mathematics, 15.04.2021 20:00








History, 15.04.2021 20:00

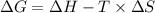
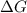 is the change in the Gibbs free energy.
is the change in the Gibbs free energy.
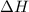 is the enthalpy change of the reaction.
is the enthalpy change of the reaction.
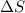 is the change in entropy.
is the change in entropy.