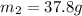Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Consider the nuclear equation below. 239 > x + 4 he 94 2 what is x? 1.235 cm 96 2.243 u 92 3.235 u 92 4.243 cm 96
Answers: 2


Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 23.06.2019 00:10
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3
You know the right answer?
A32.4 g iron rod, initially at 21.6 ∘c, is submerged into an unknown mass of water at 63.1 ∘c, in an...
Questions

Mathematics, 02.03.2021 06:00

Chemistry, 02.03.2021 06:00





Chemistry, 02.03.2021 06:00

Computers and Technology, 02.03.2021 06:00

Business, 02.03.2021 06:00

English, 02.03.2021 06:00

Social Studies, 02.03.2021 06:00

Physics, 02.03.2021 06:00

Mathematics, 02.03.2021 06:00

Mathematics, 02.03.2021 06:00

Health, 02.03.2021 06:00

History, 02.03.2021 06:00


Mathematics, 02.03.2021 06:00


Chemistry, 02.03.2021 06:00

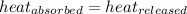
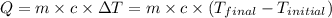
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0390/9808/09236.png) .................(1)
.................(1)
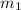 = mass of iron rod = 32.4 g
= mass of iron rod = 32.4 g
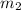 = mass of water = ?
= mass of water = ?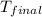 = final temperature =
= final temperature = 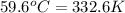
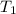 = temperature of iron rod =
= temperature of iron rod = 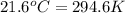
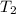 = temperature of water =
= temperature of water = 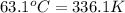
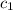 = specific heat of iron rod =
= specific heat of iron rod = 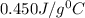
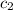 = specific heat of water=
= specific heat of water= 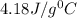
![32.4\times 0.450\times (332.6-294.6)=-[m_2\times 4.18\times (332.6-336.1)]](/tpl/images/0390/9808/cb847.png)