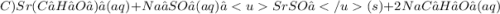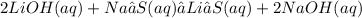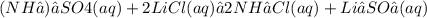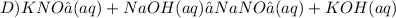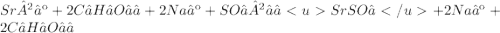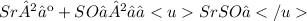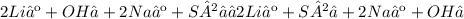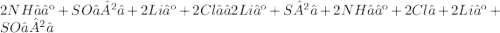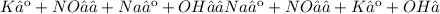Give two examples (each) of strong electrolyte, weak electrolyte, and nonelectrolyte. 2. predict the products for reactions below. which of the following reaction(s) produce a precipitate? a) lioh + na2s b) (nh4)2so4 + licl c) sr(c2h3o2)2 + na2so4 d) kno3 + naoh e) none of the above solution pairs will produce a precipitate. 3. what are the spectator ions in the precipitation reaction you chose above? 4. write the molecular, complete ionic, and net ionic equations for the reactions in q2.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Solar energy is energy from the sun that is converted into thermal or energy. a. nuclear b. mechanical c. electrical d. chemical
Answers: 2

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3
You know the right answer?
Give two examples (each) of strong electrolyte, weak electrolyte, and nonelectrolyte. 2. predict the...
Questions

Mathematics, 08.03.2021 22:00


History, 08.03.2021 22:00

Mathematics, 08.03.2021 22:00


Mathematics, 08.03.2021 22:00


Computers and Technology, 08.03.2021 22:00

Mathematics, 08.03.2021 22:00


Spanish, 08.03.2021 22:00

Mathematics, 08.03.2021 22:00

Mathematics, 08.03.2021 22:00



Mathematics, 08.03.2021 22:00

Mathematics, 08.03.2021 22:00

Mathematics, 08.03.2021 22:00


Mathematics, 08.03.2021 22:00