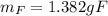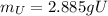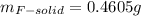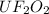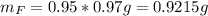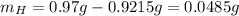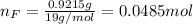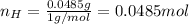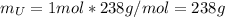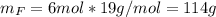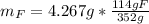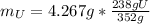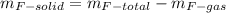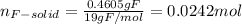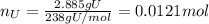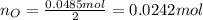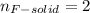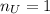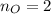Water is added to 4.267 g uf6. the only products are 3.730 g of a solid containing only uranium, oxygen and fluorine and 0.970 grams of gas. the gas is 95.0% by mass fluorine and the remainder is hydrogen.
(a) from the data, determine the molecular formula of the gas (same as the empirical formula)
(b) what is the mass of fluorine in uf6?
(c) what is the mass of uranium in uf6?
(d) what is the mass of fluorine in the solid product?
(e) determine the molecular formula (same as empirical) for the solid product the write a balanced chemical equation for the reaction.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1

Chemistry, 22.06.2019 01:30
Asap! how do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 1

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2
You know the right answer?
Water is added to 4.267 g uf6. the only products are 3.730 g of a solid containing only uranium, oxy...
Questions


Biology, 04.02.2021 16:00

Biology, 04.02.2021 16:00

Mathematics, 04.02.2021 16:00


Mathematics, 04.02.2021 16:00

Mathematics, 04.02.2021 16:00

Mathematics, 04.02.2021 16:10

Mathematics, 04.02.2021 16:10

Mathematics, 04.02.2021 16:10


Mathematics, 04.02.2021 16:10

Mathematics, 04.02.2021 16:10


Mathematics, 04.02.2021 16:10


Chemistry, 04.02.2021 16:10

Mathematics, 04.02.2021 16:10


Mathematics, 04.02.2021 16:10