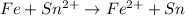Chemistry, 26.11.2019 05:31 ariannecama
Agalvanic cell with e o cell = 0.30 v can be constructed using an iron electrode in a 1.0 m fe(no3)2 solution, and either a tin electrode in a 1.0 m sn(no3)2 solution, or a chromium electrode in a 1.0 m cr(no3)3 solution even though sn2+/sn and cr3+/cr have different reduction potentials. give the overall balanced reaction for fe-sn cell. do not include the states of matter.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
One of the cell membrane's functions is to protect the cell keep wastes in the cell create new cells keep light out of the cell
Answers: 1

Chemistry, 21.06.2019 16:20
Aluminum reacts with chlorine gas to form aluminum chloride via the following reaction: 2al(s)+3cl2(g)→2alcl3(s) what is the maximum mass of aluminum chloride that can be formed when reacting 32.0 g of aluminum with 37.0 g of chlorine? express your answer to three significant figures and include the appropriate units.
Answers: 2

Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1
You know the right answer?
Agalvanic cell with e o cell = 0.30 v can be constructed using an iron electrode in a 1.0 m fe(no3)2...
Questions

Mathematics, 27.03.2021 18:50

English, 27.03.2021 18:50

Mathematics, 27.03.2021 18:50


Arts, 27.03.2021 18:50

Computers and Technology, 27.03.2021 18:50

Mathematics, 27.03.2021 18:50



Mathematics, 27.03.2021 18:50

Mathematics, 27.03.2021 18:50





Mathematics, 27.03.2021 18:50

Biology, 27.03.2021 18:50

Mathematics, 27.03.2021 18:50

Mathematics, 27.03.2021 18:50

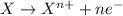
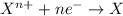

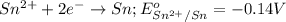
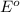 potential will always get reduced and will undergo reduction reaction. Here, zinc will always undergo reduction reaction will get reduced.
potential will always get reduced and will undergo reduction reaction. Here, zinc will always undergo reduction reaction will get reduced.