

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Joseph has hypothesized that sound travels in waves. if he were following the scientific method, what should he do next? a. ask a question. b. test the hypothesis. c. study the results. d. tell other scientists about his hypothesis.
Answers: 1

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3
You know the right answer?
Given the standard enthalpy changes for the following two reactions:
(1) n2(g) + 2o2(g)2no2(...
(1) n2(g) + 2o2(g)2no2(...
Questions

Health, 30.04.2021 21:40

Mathematics, 30.04.2021 21:40

Computers and Technology, 30.04.2021 21:40

Mathematics, 30.04.2021 21:40


English, 30.04.2021 21:50

Mathematics, 30.04.2021 21:50

English, 30.04.2021 21:50

History, 30.04.2021 21:50

History, 30.04.2021 21:50



English, 30.04.2021 21:50

Mathematics, 30.04.2021 21:50



Biology, 30.04.2021 21:50


Mathematics, 30.04.2021 21:50

 ...... ΔH° = 66.4 kJ
...... ΔH° = 66.4 kJ
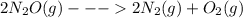 ......ΔH° = -164.2 kJ
......ΔH° = -164.2 kJ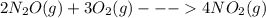 ......ΔH° = _________?
......ΔH° = _________?

