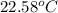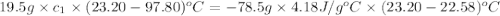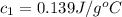Chemistry, 26.11.2019 06:31 JaleahOwens13
In the laboratory a student uses a "coffee cup" calorimeter to determine the specific heat of a metal.
she heats 19.5 grams of tungsten to 97.80°c and then drops it into a cup containing 78.3 grams of water at 22.58°c. she measures the final temperature to be 23.20°c.
assuming that all of the heat is transferred to the water, she calculates the specific heat of tungsten to be j/g°c.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1
You know the right answer?
In the laboratory a student uses a "coffee cup" calorimeter to determine the specific heat of a meta...
Questions



Geography, 10.07.2019 20:00

History, 10.07.2019 20:00

Mathematics, 10.07.2019 20:00


Health, 10.07.2019 20:00






Mathematics, 10.07.2019 20:00





Mathematics, 10.07.2019 20:00

Biology, 10.07.2019 20:00

Physics, 10.07.2019 20:00

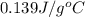
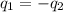
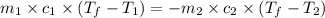
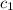 = specific heat of tungsten = ?
= specific heat of tungsten = ?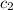 = specific heat of water =
= specific heat of water = 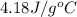
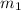 = mass of tungsten = 19.5 g
= mass of tungsten = 19.5 g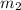 = mass of water = 78.5 g
= mass of water = 78.5 g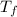 = final temperature =
= final temperature = 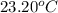
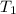 = initial temperature of tungsten =
= initial temperature of tungsten = 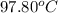
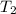 = initial temperature of water =
= initial temperature of water =