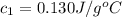Chemistry, 26.11.2019 06:31 ejhoff4347
A23.9 g sample of iridium is heated to 89.7°c, and then dropped into 20.0 g of water in a foam-cup calorimeter. the temperature of the water went from 20.1°c to 22.6°c. calculate the specific heat of iridium (specific heat of water = 4.18 j/g.°c)

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 22:30
How do limiting factors most affect population size? ostop population growthrestrict population growthincrease population sizeresult in positive impactso
Answers: 1

Chemistry, 23.06.2019 04:10
What does the field of thermodynamics relate to a-changes in nuclear reactions b- changes in energy in systems c changes in molecular structure d changes in atomic properties
Answers: 1
You know the right answer?
A23.9 g sample of iridium is heated to 89.7°c, and then dropped into 20.0 g of water in a foam-cup c...
Questions

Mathematics, 01.06.2021 19:20





Mathematics, 01.06.2021 19:20

English, 01.06.2021 19:20



Mathematics, 01.06.2021 19:20




Mathematics, 01.06.2021 19:20




Mathematics, 01.06.2021 19:20

Mathematics, 01.06.2021 19:20

Mathematics, 01.06.2021 19:20

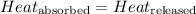
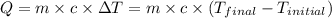
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0391/1986/09236.png) ......(1)
......(1)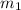 = mass of iridium = 23.9 g
= mass of iridium = 23.9 g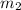 = mass of water = 20.0 g
= mass of water = 20.0 g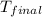 = final temperature = 22.6°C
= final temperature = 22.6°C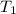 = initial temperature of iridium = 89.7°C
= initial temperature of iridium = 89.7°C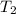 = initial temperature of water = 20.1°C
= initial temperature of water = 20.1°C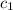 = specific heat of iridium = ?
= specific heat of iridium = ?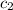 = specific heat of water = 4.18 J/g°C
= specific heat of water = 4.18 J/g°C![23.9\times c_1\times (22.6-89.7)=-[20\times 4.18\times (22.6-20.1)]](/tpl/images/0391/1986/4699c.png)